Fda Booster / Vq 7v8noz7pqhm
Moderna Asks FDA To Authorize A Booster Shot Of Its COVID-19 Vaccine. Coronavirus Updates Moderna submitted data from 344 volunteers who got a.
Two senior officials have resigned from their positions within the FDA over frustrations with the Biden administrations plans to move forward with recommending COVID-19 booster shots.
Fda booster. NYC hits 80 adult COVID jabs with surge after mandates. And last updated 2021-09-17 083730-04. Centers for Disease Control and Prevention.
Vaccine advisers to the FDA start meeting early Friday to discuss whether Americans need booster shots yet. FDA announced a virtual VRBPAC meeting to discuss the Pfizer-BioNTech supplemental application for a third booster dose of Comirnaty COVID-19 Vaccine mRNA. Today public health and.
The Federal Drug And Food Administration will. The FDA authorized Covid vaccine booster shots for people with weakened immune systems. Posted at 744 AM Sep 17 2021.
The data shows a third dose of Pfizers shot raised antibody levels with no. FDA to discuss Pfizer booster shot. Of the 289 booster recipients ages 18 to 55 monitored in Pfizers phase three trial 638 developed fatigue 484 had headaches and 391 experienced muscle pain.
Joint Statement from HHS Public Health and Medical Experts on COVID-19 Booster Shots. Booster blast Tension over boosters rises as FDA regulators quit and publicly blast Bidens plan Top FDA regulators side with WHO on boosters citing insufficient data. Such people including cancer and HIV patients in general do not produce an adequate immune response.
The FDA released documents Wednesday giving a look at Pfizers case for COVID-19 booster shots. After the FDA meets a panel of advisors to the US. 20 target to begin administering 100 million booster shots in the United States.
The FDA amended the EUAs for the Pfizer-BioNTech and Moderna COVID-19 Vaccines to allow an additional dose in certain immunocompromised individuals specifically solid organ transplant recipients. Scientists at the Food and Drug Administration FDA said Wednesday Pfizers Covid-19 vaccine seems to last long enough that a booster shot may. FDA advisory panel votes against recommending COVID-19 booster shots for most Americans Shots in the arm.
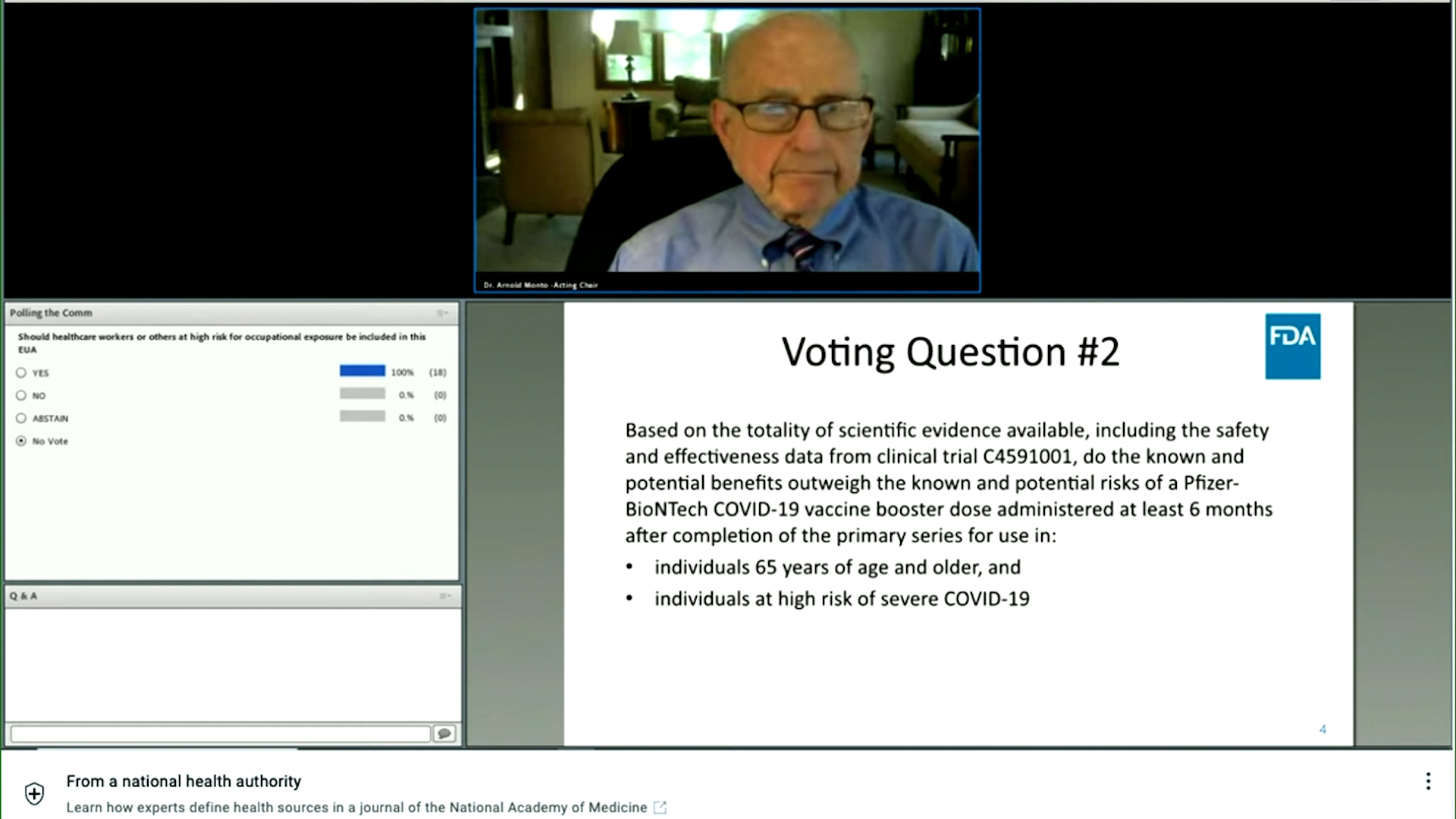
119 rijen FDA CDC and NIH are engaged in a science-based rigorous process to. Het FDA-panel keurde noodgoedkeuring goed voor de booster van het Pfizer-BioNTech COVID-19 vaccin ten minste zes maanden na de tweede dosis bij mensen van 65 jaar en ouder en mensen met een hoog risico op beroepsmatige blootstelling en ernstige COVID-19. Two high-level FDA vaccine officials quit the agency last month reportedly at least in part because President Joe Bidens administration announced plans for booster shots to start being administered on September 20 despite not having secured approval from regulators.
Biden set a Sept. The agency said observational studies dont unanimously support the suggestion that. FDA sounds skeptical note on Pfizer booster shot ahead of key vote.
FDA scientists strike skeptical tone on need for Covid-19 vaccine booster at this time likely fueling debate By Matthew Herper and Helen Branswell Sept.
Fda Releases Pfizer Covid 19 Booster Shot Data Before Panel Review

Fda Panel Issues Narrow Recommendation For Covid Vaccine Booster Doses

Fda Scientists Skeptical Of Covid 19 Booster Evidence Ahead Of Key Meeting Thehill